Analytical Reference substance
Montelukast EP Impurity E, Montelukast USP Related Compound D
1-[[[(1R)-1-[3-[(1S)-1-[[[1-(Carboxymethyl)cyclopropyl]methyl]thio]-2-(7-chloro-2-quinolinyl)ethyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl) phenyl] propyl] thio]methyl]-cyclopropaneacetic acid
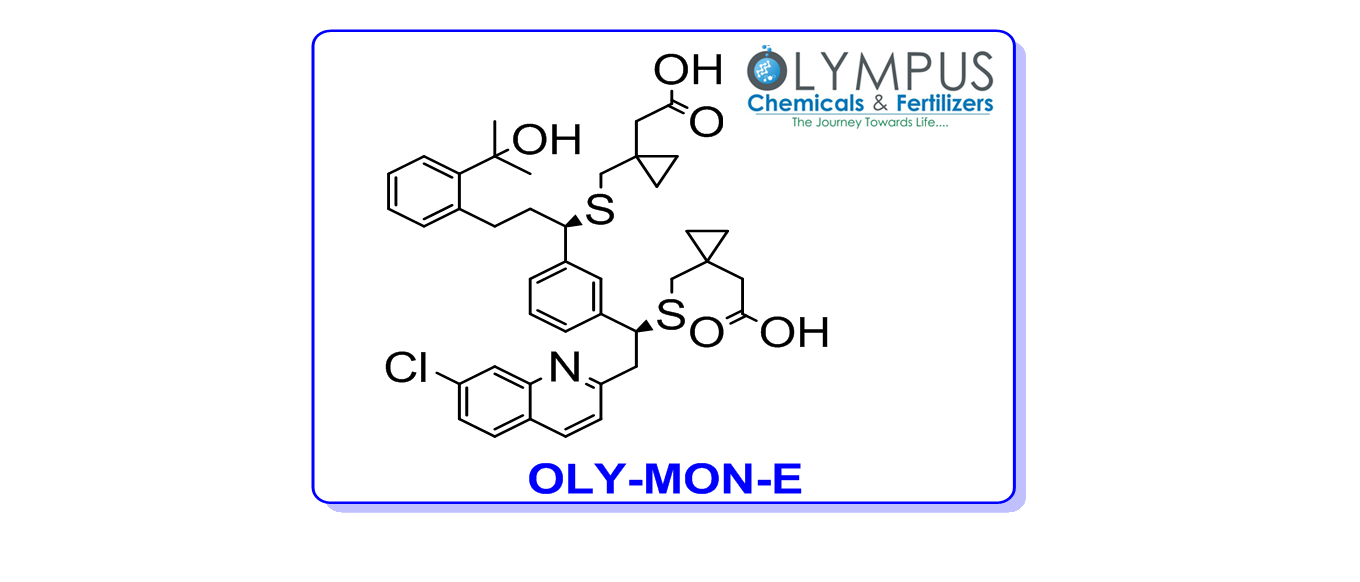
Product Number: OLY-MON-E
CAS Number: 1187586-58-8
Lot Number:
Molecular Formula: C41H46ClNO5S2
Molecular Weight: 732.39
Long Term Storage: 02-08 °C
Appearance:
Melting Point:
Purity By HPLC: NLT96
| Sr No. | Test | Results | Date ref. |
|---|---|---|---|
| 1 | 1H NMR | Confirm | Report Attached |
| 2 | Mass | Confirm | Report Attached |
| 3 | HPLC | NLT96 | Report Attached |
This certificate is valid for two years from the date of Manufacturing Provided the substance is stored under the recommended conditions.
Document data reference:
Prepared by
Dr. Ashish Keche
Head of QA & QC
Dr. Ashish Keche
Head of QA & QC
Reviewed by
Dr. Girish Hatnapure
Head of R& D
Dr. Girish Hatnapure
Head of R& D
Approved by
Dr. Atish Rodge
Managing Director
Dr. Atish Rodge
Managing Director
Corporate Office: Unit No F/10, 1st Floor Shubha Parvti Industrial Premises, Dombivali (E) Thane-421204 www.olympusimpuritiesstandard.com, +91-7506256625, Info@olympusimpuritiesstandard.com